RNA therapies and vaccines
RNA therapies for pediatric neurogenetic diseases
Recent successes in the design, delivery, and regulatory use of antisense oligonucleotide (ASO) therapies have set the stage for large-scale ASO development and deployment to treat specific mutations in individuals with neurologic diseases. Splice-switching ASOs can restore productive splicing for targeted mRNAs: the ASO therapy nusinersen targets SMN2 pre-mRNA to drive inclusion of a normally skipped exon, yielding a therapeutic increase in SMN protein levels in motor neurons of spinal muscular atrophy patients. More recently, development of an ASO to restore CLN7 mRNA splicing in a Batten disease patient progressed with unprecedented speed: from ASO design to FDA approval and patient delivery was ~11 months.
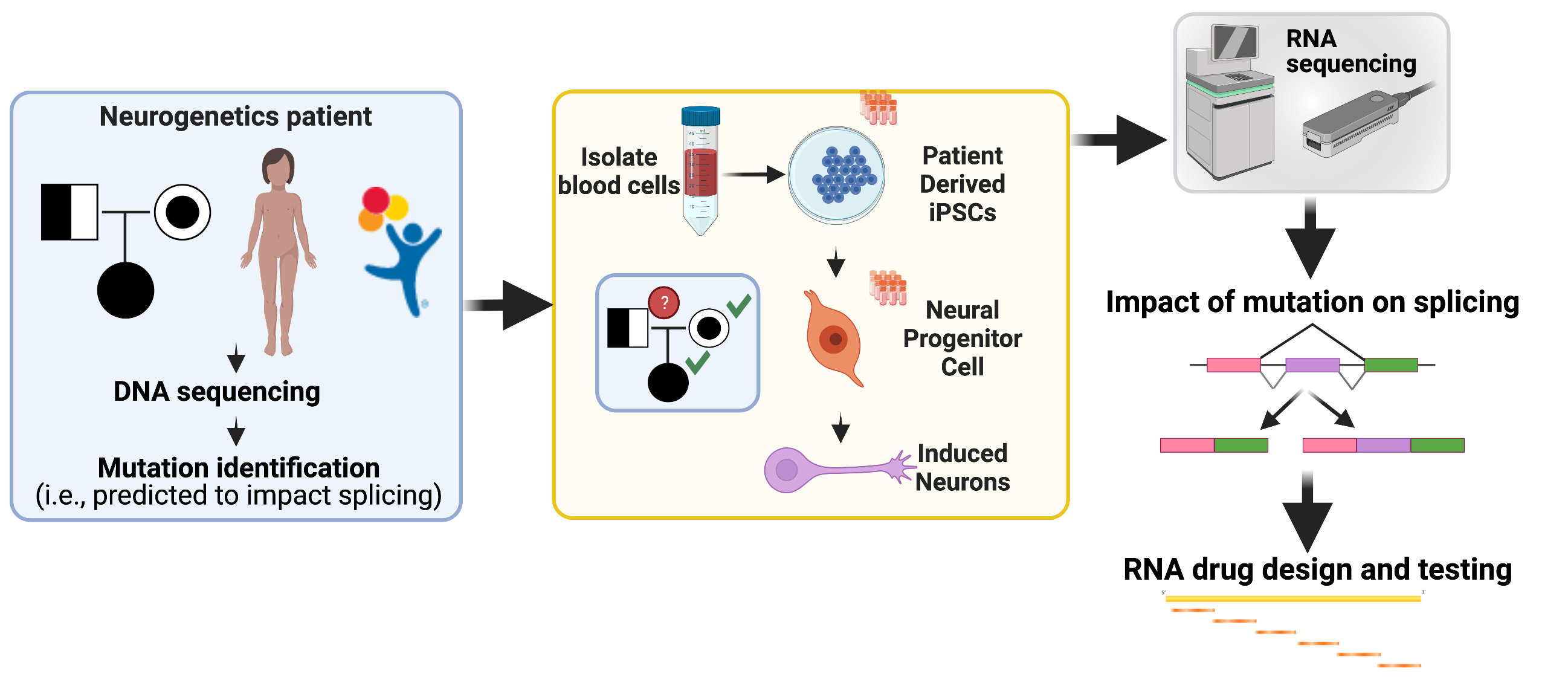
We established a pipeline with Scott Demarest, MD and the Children’s Hospital Colorado Neurogenetics Clinic to facilitate development of antisense oligonucleotide therapies for a range of neurogenetic diseases. With support of the RBI and Children’s Hospital Colorado, we create patient-derived induced pluripotent stem cell lines,
In parallel, we are exploring an alternative strategy to develop ASO modalities for diseases of haploinsufficiency that are gene-specific instead of mutation-specific. Such an approach would be transformative for the field, as it reduces the daunting challenge of individual ASO therapies to the identification of a minimal set (i.e., one for each gene) of ASOs that can target a gene irrespective of the nature of a specific mutation. To help achieve this goal, we developed a novel ASO chemistry (thiomorpholino or “TMO” modifications) with favorable characteristics for manipulating nuclear gene expression. With support from the AB Nexus program, we leverage our complementary, cross-campus expertise to compare the efficacy of TMO modifications to standard modifications (2′-methoxyethyl or “MOE”) of existing, FDA-approved therapies and leverage unique properties of TMO ASOs to manipulate nuclear mRNA expression and processing.
mRNA vaccines
The RBI initiated a pilot program to develop mRNA vaccines in collaboration wih local immunologists. We have established mRNA manufacturing and lipid nanoparticle production and have worked with several local immunologists to apply mRNA vaccines for manipulation of specific immune reponses.
Our collaborators at CU Anschutz include:
- Beth Tabmurini, PhD to apply mRNA vaccines to manipulate lymph node stromal cell populations.
- Ross Kedl, PhD to use mRNA-encoded antibodies to improve T cell-mediated immunity.
- Yuwen Zhu, PhD to develop cancer immmunotherapies.
- Linda van Dyk, PhD to develop mRNA vaccines against lymphoma-associated viruses.
Funding
- RNA Bioscience Initiative
- CU ASPIRE (with Beth Tamburini, Tem Morrison, and Jenna Guthmiller)
- NIH/NIAID R21 (with Ross Kedl)
- Cancer League of Colorado (with Linda van Dyk)
- AB Nexus (with Ondrej Kostov and Marvin Caruthers, CU Boulder)